Changes to Data Collection
Throughout the course of the study, the data collection schedule changed to accommodate sub-studies, improve collection of study endpoints and introduce new, important questions.
Changes to baseline data collection included:
- Expansion of data collection related to past medical history (June 2013)
- Expansion of data collection related to past history of cancer (June 2013)
Changes to longitudinal data collection included:
- Introduction of six-monthly administration of the six modified Katz Activity of Daily Living (ADL) questions (Feb 2013)
- Introduction of the Participant Medical History Update form (PMHU) with associated expansion of data collection related to clinical events (June 2013)
- Introduction of annual administration of CES-D 10 questionnaire (1 Jan 2015)
- Introduction of ‘current aspirin use’ questions (Milestone visit and ASPREE-XT)
- Collection of pathology dates for individual pathology measures (ASPREE-XT)
- Removal of third reading of blood pressure and heart rate (ASPREE-XT)
- Improvement in the collection of ConMed data (ASPREE-XT)
- Introduction of annual physical function measures (ASPREE-XT)
- Introduction of annual 3MS assessment (ASPREE-XT)
- Introduction of annual Color Trails assessment (XT02)
- Introduction of annual COWAT, SDMT and HVLT-R assessments (XT02)
- Introduction of phone call annual visit including cognitive assessment visit type (for participants attending in-person annual visits: Australia - Apr 2020, US - Jul 2020; for participants being followed-up via phone call only: Nov 2020 (both countries))
- Introduction of phone call 3MS reassessment visit (Nov 2020)
- Recording of Katz assessments and six-month call conducted via proxy (May 2020)
- Introduction of close out visit type (June 2020)
Changes to operational data collection included:
- Tracking of medical records only (MRO) annual visit fax attempts (Mar 2020)
- Tracking of phone call annual visit attempts (Jul 2018)
Details of these changes are discussed in the following sections.
Participant Past Medical History Collection
With the introduction of the Participant Medical History - Baseline (PMHB) form, the capture of past medical history changed from three structured medical condition fields to 17 structured medical condition fields (see Table 1 below). A free-text option to collect ‘other medical history’ remained available throughout the period of participant enrolment.
Table 1. Collection of participant past medical history pre and post June 2013.
| Participant past medical history pre June 2013 (excluding cancer) | ||
|---|---|---|
| Question | Other data | |
| History of bowel polyp | Number of polyps | |
| History of diabetes | ||
| History of kidney disease | Requirement for dialysis, history of kidney transplant | |
| History of other medical condition (free-text) | ||
| Participant past medical history post June 2013 (excluding cancer) | |
|---|---|
| Question | Other data |
| History of asthma | - |
| History of benign prostatic hyperplasia | - |
| History of bowel polyp | Number of polyps |
| History of deep vein thrombosis | - |
| History of depression | - |
| History of diabetes | - |
| History of gastro-oesophageal reflux disease | - |
| History of gout | - |
| History of high blood pressure | - |
| History of high cholesterol | - |
| History of kidney disease | Requirement for dialysis, history of kidney transplant |
| History of macular degeneration | - |
| History of minor bleeding | - |
| History of osteoarthritis | - |
| History of Parkinson’s disease | - |
| History of pneumonia | - |
| History of ringing in the ears | - |
| History of other medical condition (free-text) | - |
Following introduction of the PMHB, free-text responses under ‘other medical condition’ collected prior to June 2013 were reviewed and where appropriate converted into the new medical conditions. Medical history reports that did not fit into one the 17 new medical condition categories remained as ‘other medical condition’.
Introduction of Detailed Cancer Data Collection
The ASPREE Cancer Endpoints Study (ACES) was funded by the National Cancer Institute and operationalised in June 2013. This sub-study supported collected of new questions regarding family cancer history, participant cancer history and participant cancer screening tests which were added to data collection at baseline for participants who enrolled post-June 2013.
Family Cancer History
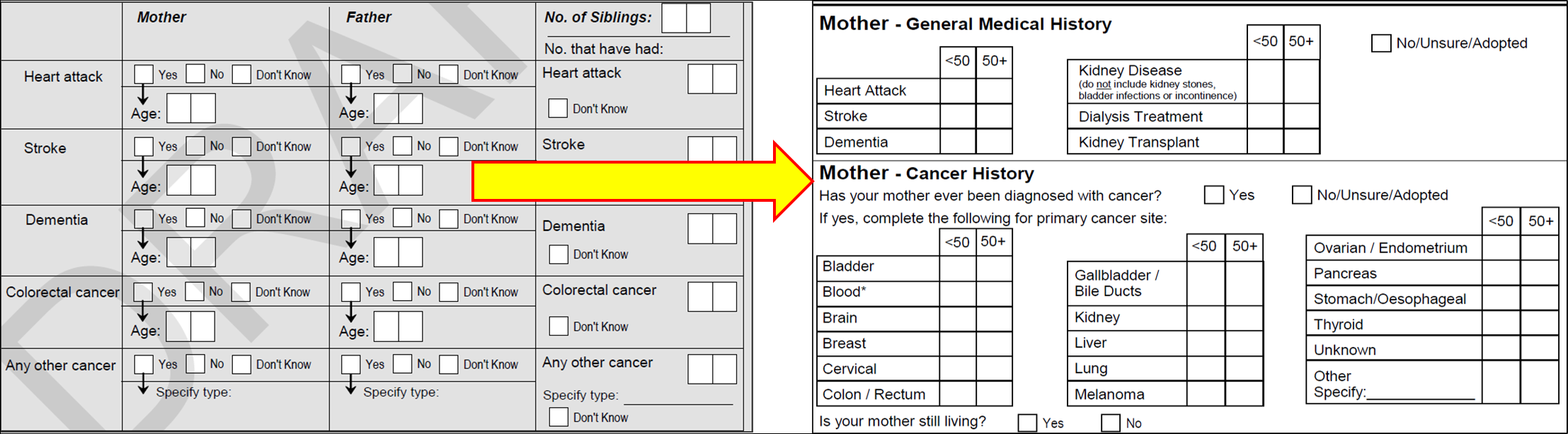
Family history questions introduced in June 2013 added to existing data regarding family cancer history. Prior to June 2013 family cancer history was collected as ‘colorectal cancer’ or ‘other cancer’. Post June 2013, a number of ‘other’ cancer types were collected. The differences in family cancer history data collection are shown below in Figure 1.
Existing free-text responses recorded under ‘other cancer’ were reviewed and, where appropriate converted, to the new detailed cancer types. Cancer reports that did not fit into a detailed cancer type remained as ‘other’. Existing data for age of onset in years was converted into ACES age brackets (< 50 years or 50+ years) for cancer onset rather than age in years.
Participant Cancer History
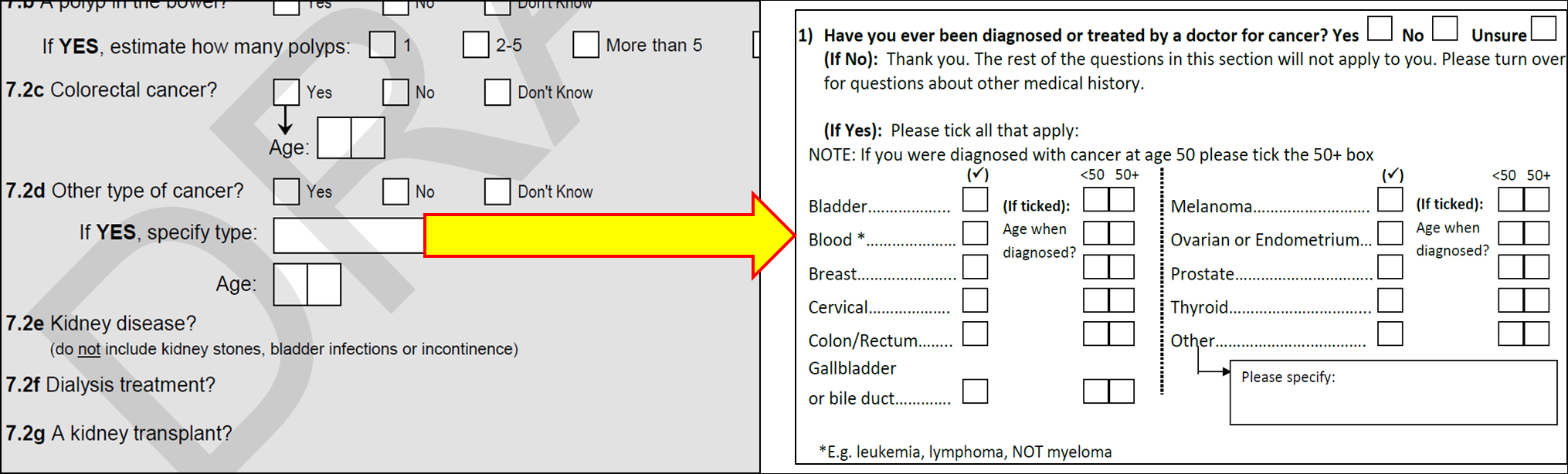
Similarly, prior to the commencement of ACES, participant cancer history was collected under the categories of ‘colorectal cancer’ or ‘other cancer’. The ACES sub-study allowed for more detailed collection of the type of pre-existing cancer. The differences in participant cancer history data collection are shown below in Figure 2.
Existing free-text responses under ‘other cancer’ were reviewed and where appropriate converted into the new detailed cancer types. Cancer reports that did not fit into a detailed cancer type remained as ‘other’. Existing data for age of onset in years was converted into ACES age brackets (< 50 years or 50+ years) for cancer onset rather than age in years.
Cancer Screening
New data was collected regarding cancer screening tests at baseline and at each annual visit. No conversion was required for these items as there was no pre-existing data.
Introduction of Modified Katz ADL Questions at all Six-Month Phone Calls
Initially, the modified Katz ADL questions were only administered at six-month phone calls only if a physical disability trigger (i.e. a report of ‘a lot of difficulty’ or ‘unable to do’ or needing assistance for one of the six Katz ADLs) was recorded at the preceding annual visit. However, in February 2013, the modified Katz ADL questions were added to six-month phone call data collection to improve detection of physical disability triggers.
Modified Katz ADL variables linked with six-month phone calls, conducted prior to February 2013, have been annotated with a commentary code of 2 to indicate that they were not active at the time of data collection.
Introduction of Participant Medical History Update
When ACES was introduced in June 2013, the system for recording clinical endpoints was updated. To ensure that staff collected sufficient information for endpoint coding and supporting document collection a new questionnaire was developed, the PMHU form. This form included structured questions about each endpoint and associated operational data.
From June 2013 onward, the PMHU was administered at six-month phone calls and annual visits for the purpose of event collection. From February 2018 onward (the start of ASPREE-XT), the PMHU was also administered via phone-call whenever a participant reported a new event of interest.
Relevant data from the PMHU has been included in Section F2 and F3 of the ASPREE Longitudinal Data Set (Version 3) and ASPREE-XT Longitudinal Data Set (XT04).
NOTE: Unverified operational fields have not been included in the data set.
Introduction of Annual CES-D 10 Questionnaire
In June 2015, the ASPREE-Depression sub-study was funded. To support this sub-study, the CES-D 10 questionnaire was introduced at all even year annual visits. Previously, the CES-D 10 was only administered at odd year annual visits, when the cognitive tests were administered.
CES-D 10 variables linked with even year visits conducted prior to June 2015 have been annotated with a commentary code of 2 to indicate that they were not active at the time of data collection.
Introduction of “Current Aspirin Use” Questions
Questions relating to current aspirin use were introduced at the Milestone visit. A similar suite of questions was captured at each six-month phone call and annual visit during ASPREE-XT. Aspirin use data captured at the Milestone visit can be found in Section G2 of the ASPREE-XT Longitudinal Data Set (XT04) and the ASPREE Longitudinal Data Set (Version 3), while aspirin use data captured during ASPREE-XT can be found in Section G3 of the ASPREE-XT Longitudinal Data Set (XT04).
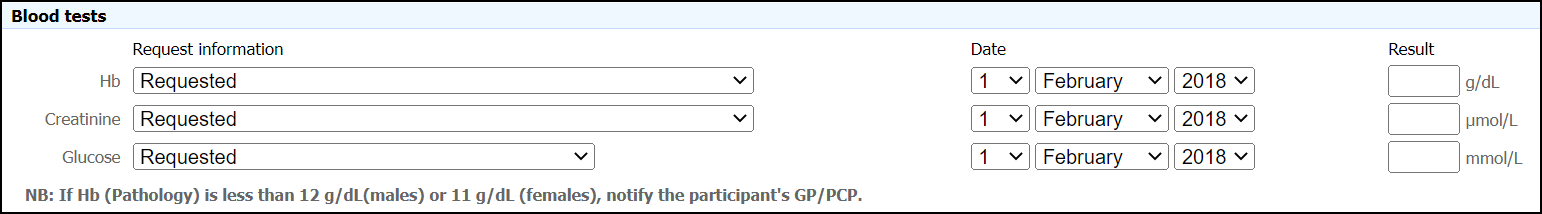
Collection of Pathology Dates
During the ASPREE Clinical Trial and Bridge period, the timing of each pathology measure was derived using the due date for each annual visit and is represented as the number of “days since randomisation” (DSR). This data is available in Section H1 of the ASPREE-XT Longitudinal Data Set (XT04) for visits conducted during this study period with variable names containing ‘Path_DSR’. For ASPREE-XT visits, data in this variable is missing and a commentary code of 3 has been applied. For ASPREE-XT visits, data in this ‘Path_DSR’ variable is missing and a commentary code of 3 has been applied. This is because ASPREE-XT pathology data was improved by collecting the specific result date for each measure (Figure 3). DSR’s specific to pathology measures are only available for XT visits i.e., from the XT01 onwards. This data is available for pathology measures captured during ASPREE-XT and can be found in Section B4 of the ASPREE-XT Longitudinal Data Set (XT04). For visits conducted prior to ASPREE-XT, these fields are blank and a commentary code of 2 has been applied.
Removal of Third Blood Pressure and Heart Rate Measurements
During the ASPREE Clinical Trial and Bridge period, mean blood pressure and mean heart rate were derived from three measurements. During ASPREE-XT, mean heart rate and mean blood pressure were calculated from only two measurements. For ASPREE-XT visits, the relevant field relating to the third measurement for each variable is blank and has a commentary code of 2 applied.
Improvement in ConMed Data Collection
During the ASPREE Clinical Trial and Bridge period, ConMed data was captured to indicate whether or not the medication was taken by the participant during each calendar year of follow-up. During ASPREE-XT, ConMed data collection was enhanced by indicating whether the participant was taking the medication at the time of the current ASPREE-XT annual visit, rather than by calendar year. See the Statistical Methods Guide for the Analysis of the ASPREE ConMed Data (1) for further information.
Introduction of Annual Assessments
During ASPREE-XT, several measures were captured annually rather than every second year, including physical function measures, the 3MS, and from XT02 onward the COWAT, SDMT and the HVLT-R.
During the ASPREE Clinical Trial and Bridge period, the Color Trails assessment was conducted during in-person dementia assessment visits and this data was not included in the ASPREE Longitudinal Data Set (Version 3). During ASPREE-XT, the Color Trails assessment was conducted every year from the XT02 visit onward and these data have been included in Section C2 of the ASPREE-XT Longitudinal Data Set (XT04).
Introduction of Phone Call Cognitive Assessments
Due to the impact of COVID-19 on our ability to conduct in-person visits throughout 2019 and 2020, the existing phone call annual visit was modified to enable a comprehensive cognitive battery to be delivered over the phone (called a ‘phone call + cogs’ visit). These visits included all standard questionnaires regularly associated with an annual phone call visit, with the addition of the 3MS, COWAT and HVLT-R cognitive assessments modified to accommodate administration by telephone. The mode in which a visit was conducted is indicated in the ‘visit conduct’ variable in Section H1 of the ASPREE-XT Longitudinal Data Set (XT04).
To highlight the difference in overall maximum possible score when the 3MS is conducted in-person (100) and via phone (74), the overall score has been provided in two distinct variables in Section C1. AVx_3MS_OverallScore represents the overall score when the 3MS was conducted in-person. AVx_3MS_OverallScore_Phone represents the overall score when the 3MS was conducted via phone.
3MS reassessment visits can now also be conducted over the phone. A new ‘visit conduct’ variable is provided for each 3MS reassessment visit in Section H1 to indicate whether the reassessment took place in-person or via phone call. Overall scores for reassessment visits conducted in-person and via phone have also been presented in two separate variables (AVx_Reassess_3MS_OverallScore and AVx_Reassess_3MS_OverallScore_Phone).
The phone call cognitive assessment visit type will continue to be available for situations where we would otherwise be unable to collect cognitive data e.g. participant has requested phone call follow-up only. Wherever possible, in-person conduct of the battery of cognitive assessments will be prioritised.
Introduction of Phone Call Cognitive Assessments
During ASPREE-XT, AWARD-Data was modified to record in a structured format whether Katz assessment questions were conducted via a proxy. To account for administration of the ADLs via proxy at an annual visit or six-month phone call, the ‘visit conduct’ categories for these visit types were updated in Section H1 of the ASPREE-XT Longitudinal Data Set (XT04). An ‘interview completed’ variable for each Katz assessment was also added to Section E1 of the ASPREE-XT Longitudinal Data Set (XT04). The conduct of a Katz reassessment by proxy was recorded in a structured format from May 2020.
Prior to these updates, staff recorded whether the questions were conducted via proxy in a free-text field. This data has not been included in this data set and as such, it is not possible to distinguish visits where the Katz ADL questions have been conducted with the participant from those conducted via proxy in this period. ‘Interview completed’ variables for Katz assessments conducted during the ASPREE Clinical Trial have a commentary code of 2 applied.
Similarly, during ASPREE-XT, AWARD-Data was modified to record if a six-month phone call was completed via a suitable proxy. If the call was completed via proxy, part two of the PMHU along with the aspirin use questions were not completed, as it was deemed that a proxy may not have full knowledge of this information. A new ‘visit conduct’ category has been created in Section H1 of the ASPREE-XT Longitudinal Data Set (XT04) to indicate if a six-month phone call was conducted via a suitable proxy.
Introduction of Close Out Visit
Operational changes were implemented to support the conduct of a final MRO visit following the death of a participant to collect any final relevant medical records including ConMed data, pathology and hospitalisations for reasons other than endpoints. In Australia, the close out visit was conducted approximately three months following the death of a participant to allow adequate time for supporting documents to reach the participant’s clinic. The PMHU was also completed at each close out visit and any relevant medical records collected to support any new events of interest. Data collected at close out visits can be found in dedicated ‘CO’ variables within each relevant section in the ASPREE-XT Longitudinal Data Set (XT04).
Tracking of MRO Annual Visit Fax Attempts and Phone Call Annual Visit Attempts
For MRO participants, if staff were unable to visit the GP clinic to collect medical records for an annual visit, medical records were requested from the participant’s GP via fax. To streamline this process, the number of fax request attempts (maximum number of three attempts) was tracked during ASPREE-XT from March 2020 onward. Similarly, for annual visits conducted via phone call, the number of phone call attempts was tracked during ASPREE-XT from July 2018 onward. For annual visits that were possible but not completed, i.e. the visit DSR is missing, there are two possible commentary codes. If the number of attempts for the visit was NOT reached, a commentary code of 12 was applied. If the maximum number of attempts for a visit was reached, a commentary code of 27 was applied.
References
- Broder J and Wolfe RS (2021) ‘ConMeds statistical method guidelines v1.0’, School of Public Health and Preventive Medicine, Monash University, Melbourne.