Clinical Event Coding
An overview of the endpoint confirmation process, for the ASPREE Clinical Trial, the Bridge period and ASPREE-XT, is shown in Figure 1. Collection, coding, follow up and adjudication of endpoint triggers was tracked via AWARD-Data and AWARD-Adjudicator.
Detailed information about event coding, supporting document collection and adjudication are included below.
Event Coding
Following entry of a clinical event into AWARD-Data, each event was reviewed and coded by a central team at the Australian National Coordinating Centre. Coding involved either allocating the event to the correct adjudication committee (by clinical event type) to review the case, marking the case as a duplicate, or indicating the event was not an endpoint. Detailed Standard Operating Procedures (SOPs) were provided to event coders to follow.
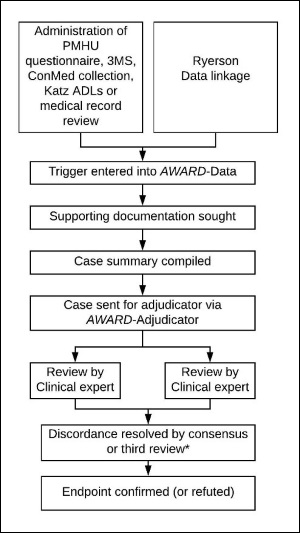
At the coding step, operational details were recorded in AWARD-Data to facilitate collection of supporting documentation (described below in Event Supporting Documentation) and an analytical ‘Endpoint Date’ was entered. The date entered into this field was based on standard criteria (see Table 1). The endpoint date was reviewed once all supporting documentation was collected to ensure that the correct date was recorded according to the criteria. The endpoint date is used to calculate the event “days since randomisation” (DSR).
Table 1: Criteria for ‘Endpoint Date’ by endpoint and event type.
| Endpoint | Event Type | Endpoint Date |
|---|---|---|
| Cancer | Non-metastatic cancer | Date of histopathological confirmation |
| Metastatic cancer | Date of confirmed metastasis | |
| Clinically Significant Bleeding | Non-fatal bleed | Date of 1st hosp; transfusion, surgery |
| Fatal bleed | Date of death | |
| Death | Any death | Date of death |
| Dementia | 3MS trigger | Date of 3MS trigger |
| ConMed trigger | Date of ConMed prescription | |
| Diagnosis trigger | Date of diagnosis | |
| Depression | High CES-D 10 score | Date of CES-D 10 administration |
| Hospitalisation for depression | Date of admission | |
| Hospitalisation for Heart Failure | All | Date of hospitalisation |
| Mild Cognitive Impairment | N/A not coded or adjudicated | - |
| Myocardial Infarction | Acute MI | Date of high troponin or date of death |
| Established MI | Date of new pathological Q waves or date of death | |
| Physical Disability | Persistent loss of Katz ADL | Date of first Katz loss that was later confirmed to be persistent |
| Admission to care | Date of ACAS report | |
| Stroke | Any | Date of symptoms |
Event Supporting Documentation
Supporting documentation was sought for all primary and secondary endpoints and cause of death, based on the evidence required to meet the endpoint definitions outlined in the ASPREE Clinical Trial and ASPREE-XT protocols. Tables 2 and 3 detail the documentation usually sought by event type and protocol criterion.
Table 2. Data required and linked supporting documentation for components of the primary outcome.
| Event | Evidence required to meet ASPREE criteria | Document that may contain data required |
|---|---|---|
| Death | 1. Confirmation of death | Death certification or verification from state-based Births, Deaths and Marriages |
| 2. Date of death | As above | |
| Dementia | 1. Cognitive decline in two domains | ASPREE cognitive assessments; Aged Care Assessment Service report; Specialist letter |
| 2. Functional decline | ASPREE function assessments e.g. IADL; Aged Care Assessment Service report; Specialist letter; General Practitioner (GP)/Primary care provider (PCP) correspondence | |
| Physical Disability - Admission to Care | 1. Admission to care for assistance with ADLs due to a physical disability | Aged Care Assessment Service report |
Table 3. Data required and linked supporting documentation for secondary endpoints.
| Event | Evidence required to meet ASPREE criteria | Document that may contain data required |
|---|---|---|
| Cancer | 1. Presence of cancer | Histopathology report; CT/MRI/PET scan |
| 2. Progression of cancer | Clinical notes (hospital or GP/PCP/Specialist); CT/MRI/PET scan | |
| Clinically Significant Bleeding | 1. Substantiated bleeding | Discharge summary; Clinical notes (hospital or GP/PCP/Specialist) |
| 2. Hospitalisation, surgery, transfusion or death (from bleeding) | Discharge summary; Operation report; Transfusion record (for packed red blood cells (PRBCs)) | |
| Hospitalisation for Depression | 1. Depression as primary diagnosis | Discharge summary |
| Hospitalisation for Heart Failure | 1. Heart failure on admission | Clinical notes (hospital or GP/PCP/Specialist); Discharge summary |
| 2. Admission for > 24 hours | Discharge summary | |
| Myocardial Infarction | 1. Troponin rise PLUS* | Pathology report |
| 2. *PLUS = one of: ischaemic symptoms, ECG changes, coronary artery intervention | Discharge summary; ECG; Clinical notes (hospital or GP/PCP/Specialist) | |
| 3. Pathological changes of MI | Coroner’s report | |
| Stroke | 1. Symptoms of stroke | Discharge summary; Clinical notes (hospital or GP/PCP/Specialist); Specialist letter |
| 2. Event duration of > 24 hours | Discharge summary | |
| 3. Confirmation on imaging | MRI/CT scan | |
In Australia, supporting documentation was collected from General Practitioner (GP) practices during the annual medical record review. A central team at the Australian National Coordinating Centre also directly requested documentation from hospitals and specialists.
In the US, supporting documentation was collected by site staff according to local protocols. In both countries, any supporting documentation obtained was uploaded to AWARD-Data and then reviewed centrally.
Following upload of sufficient documentation (according to evidence requirements from the relevant ASPREE Protocol) or confirmation that no further documentation was available, a clinical case summary document was compiled for each event. This summary document included copies of all relevant supporting documentation, and outlined the relevant history of the disease and the participant’s significant study dates (e.g. dates of randomisation and annual visits). Each summary was uploaded to AWARD-Data prior to adjudication.
Please note, the clinical case summary document was a PDF file, which is an unstructured data format. The ASPREE-XT Longitudinal Data Set (XT04) and the ASPREE Longitudinal Data Set (Version 3) include only structured data. For most endpoints, this means that the data set includes the timing of the event (expressed by a DSR), the adjudicated event type, and adjudicated sub-type. Unstructured data (such as PDF records) are not included in the data set and are not publicly available.